UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its Charter)
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On November 10, 2021, Acumen Pharmaceuticals, Inc. (the “Company”) will post a presentation regarding ACU193, a monoclonal antibody that selectively targets toxic amyloid-beta oligomers for the potential treatment of early Alzheimer’s disease, and the trial design of the Company’s ongoing Phase 1 clinical trial, INTERCEPT-AD, to its website at https://investors.acumenpharm.com/news-events/presentations, which the Company may use from time to time in communications or conferences. A copy of the presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K (this “Report”).
The information in this Report, including Exhibit 99.1 hereto, is furnished pursuant to Item 7.01 and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company’s submission of this Report shall not be deemed an admission as to the materiality of any information required to be disclosed solely to satisfy the requirements of Regulation FD.
This Report and Exhibit 99.1 hereto contain forward-looking statements within the meaning of the federal securities laws. These forward-looking statements are based on current expectations and are not guarantees of future performance. Further, the forward-looking statements are subject to limitations listed in Exhibit 99.1 and in the other reports of the Company filed with the Securities and Exchange Commission, including that actual events or results may differ materially from those in the forward-looking statements.
Item 9.01 Financial Statements and Exhibits.
| (d) | Exhibits |
| Exhibit No. |
Description | |
| 99.1 | Overview of ACU193 and INTERCEPT-AD Presentation, dated November 2021 | |
| 104 | Cover Page Interactive Data File (the cover page XBRL tags are embedded within the inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Acumen Pharmaceuticals, Inc. | ||||||
| Dated: November 10, 2021 | By: | /s/ Matthew Zuga | ||||
| Matthew Zuga | ||||||
| Chief Financial Officer and Chief Business Officer | ||||||

Overview of product candidate ACU193 and the ongoing Phase 1 INTERCEPT-AD trial November 2021 Exhibit 99.1

FORWARD-LOOKING STATEMENTS AND NOTES REGARDING THIS PRESENTATION This presentation may contain forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Any statement describing Acumen’s goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Words such as “believes,” “expects,” “anticipates,” “could,” “would,” “seeks,” “aims,” “plans,” “potential”, “will” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements include statements concerning Acumen’s business and the therapeutic potential of Acumen’s product candidate, ACU193, including its potential for improved safety and efficacy as compared to other monoclonal antibodies in development, as well as the expectations concerning the INTERCEPT-AD trial. These statements are based upon the current beliefs and expectations of Acumen management, and are subject to certain factors, risks and uncertainties, particularly those inherent in the process of discovering, developing and commercializing safe and effective human therapeutics. Such risks may be amplified by the impacts of the COVID-19 pandemic. These and other risks concerning Acumen’s programs are described in additional detail in Acumen’s filings with the Securities and Exchange Commission (“SEC”), including in Acumen’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021, filed with the SEC on August 16, 2021, which is available on the SEC’s website at www.sec.gov. Copies of these and other documents are available from Acumen. Additional information will be made available in other filings that Acumen makes from time to time with the SEC. These forward-looking statements speak only as of the date hereof, and Acumen expressly disclaims any obligation to update or revise any forward-looking statement, except as otherwise required by law, whether, as a result of new information, future events or otherwise. This presentation discusses Acumen’s investigational drug ACU193 that is in an early Phase 1 First in Humans clinical study. ACU193 has not been approved for marketing by the U.S. Food and Drug Administration or any regulatory authority. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Acumen’s own internal estimates and research. While Acumen believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

AbOs may consist of 2 to >200 Ab peptides. Kelley et al. Simulating oligomerization at experimental concentrations and long timescales: A Markov state model approach. J Chem Physics 2008. Quaternary structures of Aβ Oligomers, protofibrils and fibrils Relini et al. Misfolding of amyloidogenic proteins and their interactions with membranes Biomolecules 2014 Figure 3. Atomic force microscopy images of representative steps of amyloid aggregation: (A) oligomers; (B) protofibrils; (C) mature fibrils. Scan size 1.0 µm. Z range (A) 8.0 nm; (B) 15 nm; (C) 20 nm.

Even in the presence of a large excess of Aβ monomer, binding of ACU193 to AβOs is unchanged Highly selective for Aβ oligomers versus Aβ monomers ACU193 is the first mAb developed to selectively target AβOs Binding of ACU193 to AβOs >500x binding to Aβ monomer SELECTIVITY ACU193 selective binding to AbOs is preserved even in the presence of a large excess of Ab monomer Log [Competing Antigen] μM ACU193 Selectivity ACU193 Selectivity in presence of 5μM monomeric Aβ Data On File % Percent Control AbOs binding (RLUs) ACU193 Log μM

AD Hippocampus ThioS/amyloid plaque AD Hippocampus ACU193/AbOs ACU193 is highly selective for AβOs versus Aβ plaques ACU193 staining in human AD brain slices from hippocampus: ACU193 (red) binds non-Thioflavin S positive Ab (green) SELECTIVITY ACU193 has limited to no binding to thioflavin S positive fibrillar Aβ plaque in human AD brain tissue Cline E. et al. Synaptic intervention in Alzheimer’s disease: soluble Aβ oligomer directed ACU193 monoclonal antibody therapeutic for treatment of early Alzheimer’s disease. J. Prevent. Alzheimer’s Dis., 6 (Supplement 1) (2019), p. S151.
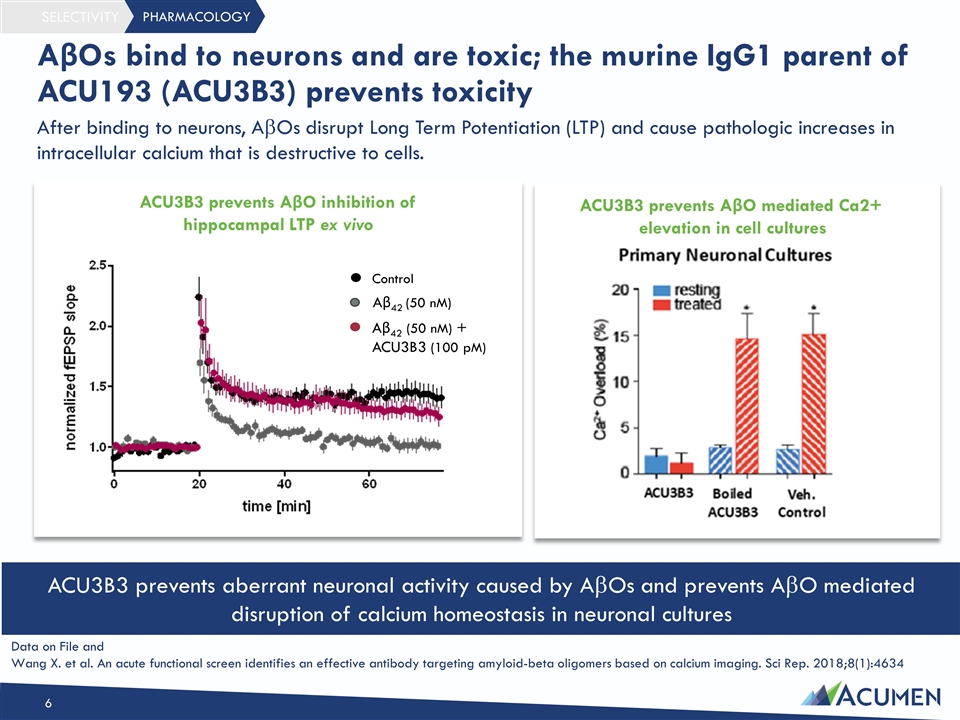
PHARMACOLOGY SELECTIVITY After binding to neurons, AbOs disrupt Long Term Potentiation (LTP) and cause pathologic increases in intracellular calcium that is destructive to cells. AβOs bind to neurons and are toxic; the murine IgG1 parent of ACU193 (ACU3B3) prevents toxicity ACU3B3 prevents AβO inhibition of hippocampal LTP ex vivo Control Aβ42 (50 nM) Aβ42 (50 nM) + ACU3B3 (100 pM) ACU3B3 prevents AβO mediated Ca2+ elevation in cell cultures ACU3B3 prevents aberrant neuronal activity caused by AbOs and prevents AbO mediated disruption of calcium homeostasis in neuronal cultures Data on File and Wang X. et al. An acute functional screen identifies an effective antibody targeting amyloid-beta oligomers based on calcium imaging. Sci Rep. 2018;8(1):4634

Murine parent of ACU193 (3B3) was used to treat younger mice with depositing plaque or older mice with abundant plaque Treatment of a transgenic mouse model of AD results in reduction of behavioral deficits Deficits in younger (5-7 months) transgenic mice are markedly reduced with treatment PHARMACOLOGY SELECTIVITY Morris Water Maze Deficits in older (9-10 months) transgenic mice are markedly reduced with treatment MWM swim speed abnormality (**p<0.02). Open field total distance measurement, APP-Veh vs. APP-3B3, *p=0.029. Open Field Dodart JC et al. Passive immunization with the anti-Aβ oligomer antibody ACU-3B3 improves behavioral deficits in hAPPSL tg mice. SfN, Washington, DC 2014. Ma K. et al. Soluble Aβ-Oligomer–Selective Antibody ACU3B3 Reduces Amyloid Pathology & Improves Multiple Behavioral Domains in a Mouse Model of AD. Alz&Dement.15, (7S Part 11 P2-063).1Jul2019 and Data on File

Phase 1 overview TRIAL DESIGN: Randomized Placebo Controlled Phase 1 Part A : Single-Ascending Dose Part B : Multiple-Ascending Doses ENROLLMENT CRITERIA: Early AD Mild Cognitive Impairment and Mild Dementia due to AD (amyloid positive by PET) TRIAL OBJECTIVES: Proof of Mechanism (PoM) Safety and tolerability Pharmacokinetics Target Engagement Exploratory cognition and biomarkers

Randomized Placebo Controlled Phase 1 in Early AD patients: INTERCEPT-AD PART A: SINGLE- ASCENDING DOSE n = 8 per cohort (32 total) PART B: MULTIPLE- ASCENDING DOSE n = 10 per cohort (30 total) COHORT 1: 2 mg/kg ACU193 or Placebo 2mg COHORT 2: 10 mg/kg ACU193 or Placebo 10mg COHORT 3: 25 mg/kg ACU193 or Placebo 25mg COHORT 4: 60 mg/kg ACU193 or Placebo 60mg COHORT 5: 10 mg/kg ACU193 or Placebo (Q4W) 10mg COHORT 6: 60 mg/kg ACU193 or Placebo (Q4W) 60mg COHORT 7: 60 mg/kg ACU193 or Placebo (Q2W) 60mg ≥ 1wk ≥ 1wk ≥ 1wk ≥ 1wk ≥ 1wk ≥ 4wk NCT04931459

Cogstate computerized test battery Test Domains tested Time (minutes) International shopping list test (immediate) Immediate recall 5 Cogstate brief battery Attention, working memory, learning 15 International shopping list test (delayed) Delayed recall 1 Groton maze learning test Executive function 7 International digit-symbol substitution test Processing speed 3 Total = 31 Frequency of administration and sensitivity of battery offers improved possibility to observe effects

Arterial Spin Labelling (ASL) as an MRI outcome MCI patients show hypoperfusion in parietal cortex, precuneus, posterior cingulate cortex and medial temporal lobe AD patients show global hypoperfusion, but especially cingulate, precuneus, parietal lobes and inferior frontal regions Perfusion correlates with several neuropsychological tests Hypoperfusion can be improved in middle and posterior cingulate cortex with cholinesterase inhibitors and was associated with improvement in ADAS-cog scores Acumen believes additional literature supports use of ASL to assess hypoperfusion in AD * N. Zhang et al. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neuroscience and Behavioral Reviews 72 (2017) 168-175 *


Summary Non-clinical data consistent with toxicity of Ab oligomers and selective binding of ACU193 to Ab oligomers Enrollment in a Phase 1study assessing safety and target engagement is ongoing Although unlikely with this small sample size, the possibility of improvement in cognition and cerebral blood flow will also be assessed as exploratory outcomes in the Phase 1 study

Thank you! Study participants and study partners ACU-001 sites Acumen collaborators
